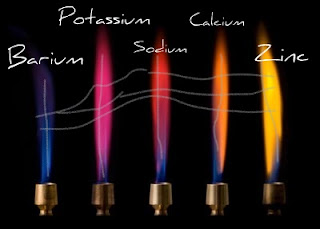
Flame test is used to determine the elements present in a compound, because every element emits unique color of the flame which is used to identify the elements. Below are some elements with their corresponding flame color.
boron -green
calcium - orange
sodium - yellow orange
potassium - light violet
copper - blue-green
barium - pale green
lithium - red
magnesium - white sparks
iron - yellow sparks
arsenic - blue
cesium - blue
copper (I) - green
strontium - crimson
thallium - pure green
How does the flame color produced?
How does the color of the flame produced? What happens inside the metal atom? As the metal atom is heated the electrons outside the nucleus are the ones that receive the heat, electrons then jump to higher energy level upon absorption of heat. As the electron jumps to higher energy level, the electron is said to be in the excited state, and in that state the electron becomes unstable, the tendency the electron will go back to lower energy levels emitting the excess energy in the form of light. And this was the basis of Neil's Bohr Model of an atom.

No comments:
Post a Comment